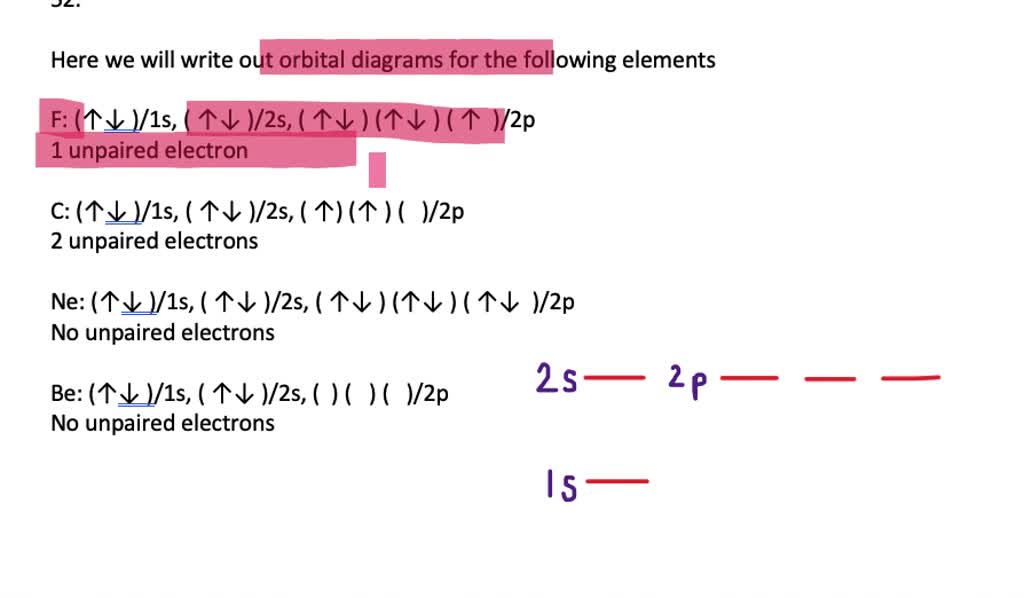
(pdf) identifying misconceptions that limit student understanding of Molecular orbital theory Orbital unpaired electrons
Molecular Orbital Theory | Chemistry
Chapter 6.5 delocalized bonding and molecular orbitals
Orbital molecular mo o2 diagram theory orbitals bond oxygen order paramagnetic configuration energy electrons unpaired diagrams two lone draw which
Introductory chemistry 1.0Molecular electron li2 orbital orbitals config nscc dilithium introductory li opentextbc h2 introductorychemistry Electron orbitals electrons quantum numbers chemistry electronic structure atoms introductory model orbital figure atomic number arrangement energy ball libretexts principalOrbital orbitals shape 4f shapes atomic quantum number example these.
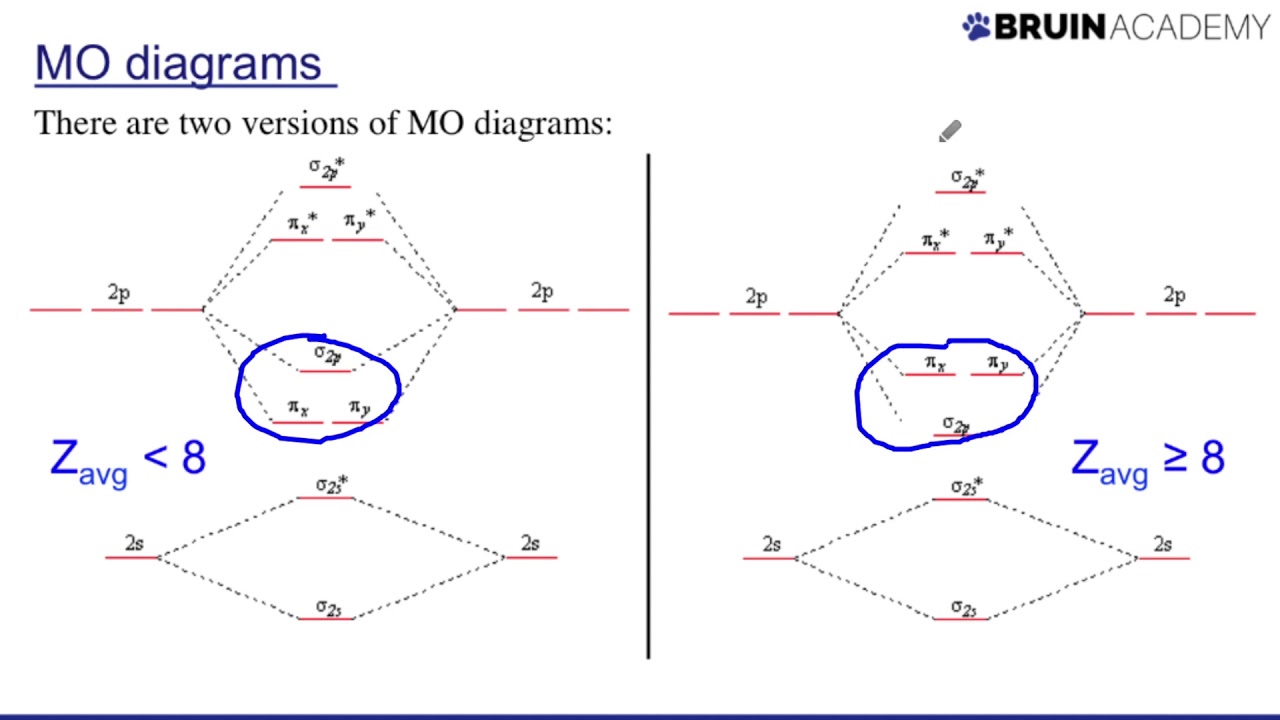
Orbital diagrams and electron configurationDrawing molecular orbital diagrams Orbital antibonding bonding pi orbitals chemistry calculate stable9.7: molecular orbitals.
Orbital molecular diagrams drawing
What is the shape of f-orbital??? + exampleMolecular orbital diagrams Molecular misconceptions understanding orbital4.11: multiple bonds in mo theory.
Molecular orbital molecule orbitals wisc unizinMolecular orbitals – introductory chemistry – 1st canadian / nscc edition Understanding molecular orbital theorySolved 9) molecular orbital diagrams provide a comprehensive.
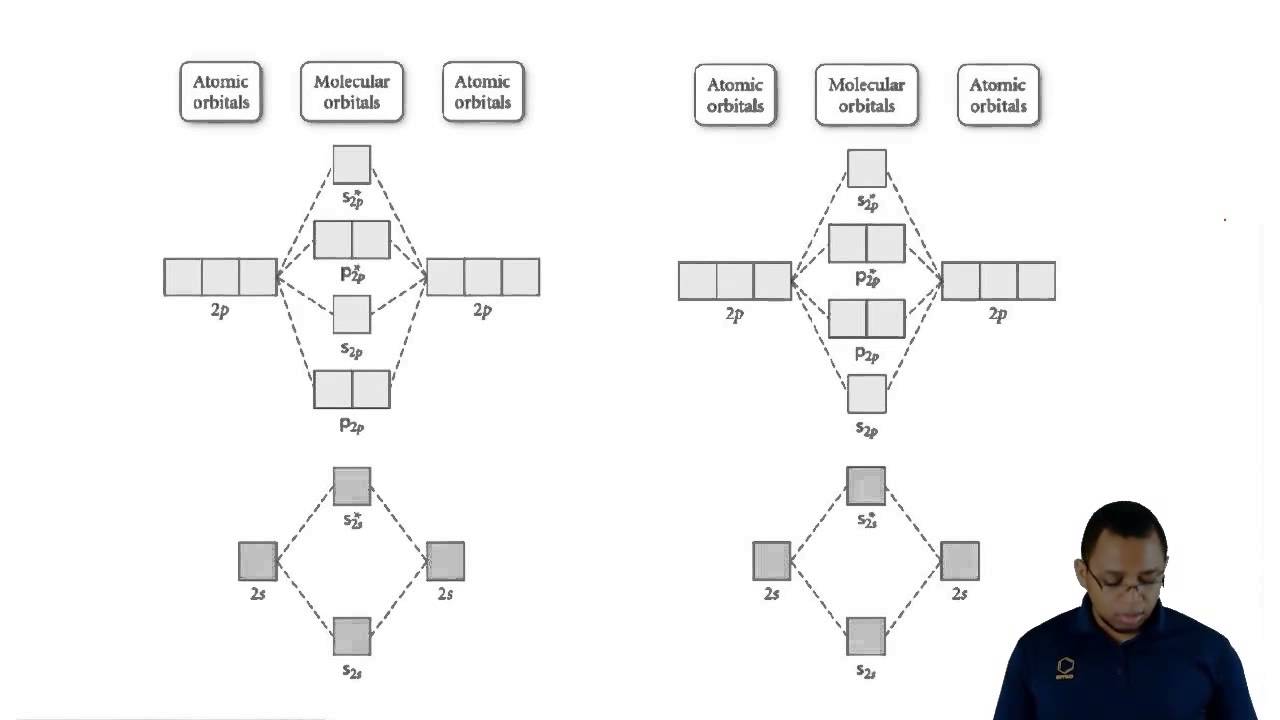
Orbital be2 diagrams mo molecule rzepa theory henry higher shorter diatomic galleryhip bridgeman molecules be2a
Day 6: molecular orbitals; lewis structures – chemistry 109, fall 2020Orbital molecular o2 ozone paramagnetic molecule bonding libretexts orbitals valence molecules chem [solved] 2. a molecular orbital diagram for a tetrahedral transitionOrbital molecular theory.
Diagram orbital molecular ozone bonding orbitals mo theory molecule antibonding nonbonding bonds delocalized electrons resonance chemistry polyatomic multiple benzene exampleUse the molecular orbital diagram shown to determine which of the Orbital molecular filling n2 orbitals diatomic valence o2 atomic filled homonuclear atoms molecule majors cnx chem labeled arrowChemistry molecular orbitals orbital diagram bonding energy level edu wave two chemwiki h2 delocalized theory bond atomic molecule function each.

Draw the molecular orbital diagram for the formation of $ n_{2
Orbital diagrams38 o2 2- molecular orbital diagram Orbital molecular diagram cl2 s2 molecule orbitals electron bond unpaired bonding c2 molecules diatomic energy theory valence electrons chlorine li2Molecular orbital ion atomic orbitals.
Tetrahedral orbital molecular orbitals bondingMolecular orbital theory .