Normal boiling point on phase diagram Point graph boiling freezing phase change curve diagram cooling heating aka presentation ppt powerpoint Point phase boiling diagram normal matter condensed chem clock states
Normal Boiling Point On Phase Diagram - General Wiring Diagram
Phase diagrams
Boiling point diagram pvt example
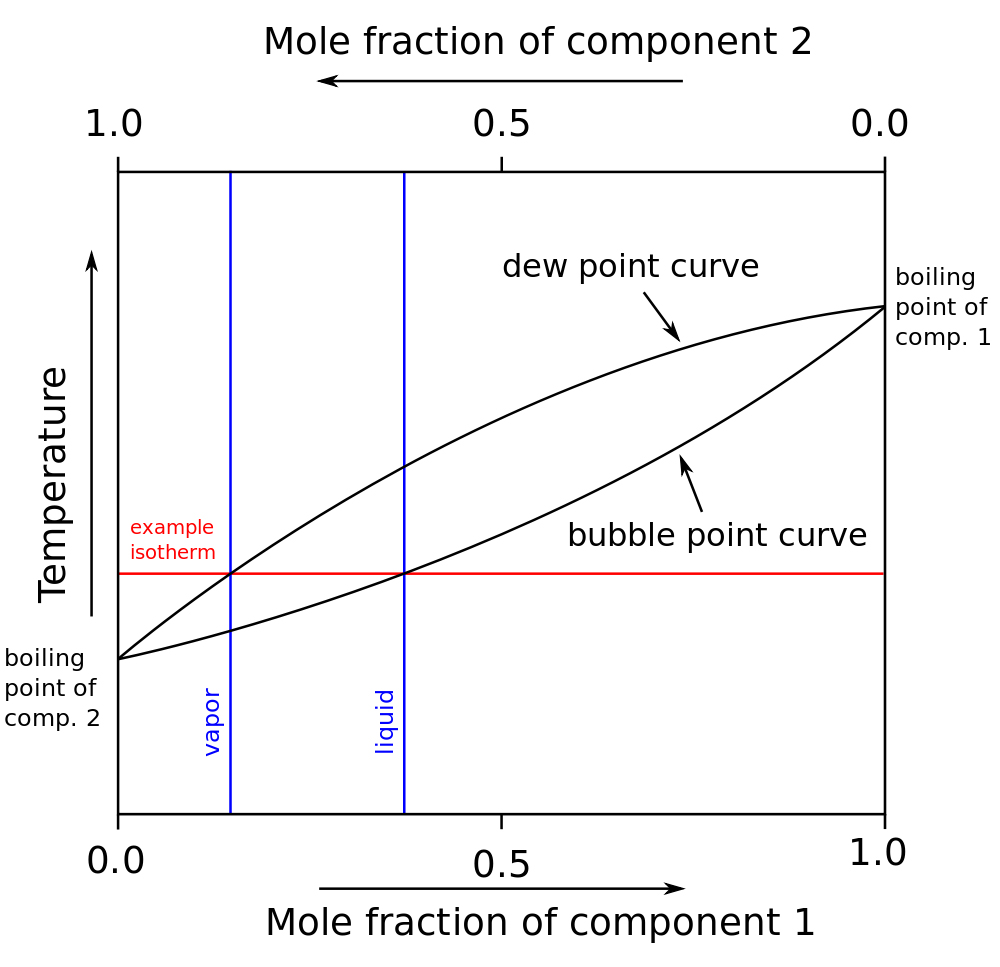
Freezing solvent depression elevation boiling equilibriumProve that the freezing point of water is 0 and the boiling point of Chemistry 4 students: boiling-point-composition diagramWater diagrams thinglink 101diagrams.
Heating phase curves curve water temperature heat graph diagram pressure change boiling liquid gas line labeled point ice diagrams chemistryBoiling point from pvt diagram (example) 7 best images of energy phase change worksheetPh and equilibrium.
1.5 phase changes – university physics volume 2
Graph point boiling phase change worksheet diagram answer energy key zing freezing worksheeto viaPhase pure pressure diagrams temperature solid liquid if higher substances melting line between will would other turn Phase diagramsArgon thermodynamic boiling vapor entropy vaporization boundary socratic behavior melting occurs gpa.
Graph triple sublimation boiling curve versus melting degrees theory celsius unexplained mysteriesPhase change Boiling following clutch prep answerBoiling change phase water lab melting freezing line condensation graphs evaporation 8th science weebly below.

Normal boiling point on phase diagram
Calculate the standard entropy of vaporization of argon at its normalTemperature vapor boiling socratic Heating curves and phase diagrams (m11q2) – uw-madison chemistry 103/Ice curve heating boiling point water temperature phase diagram energy when heat why time plot solid do science difference into.
Phase change diagramsBoiling point composition diagram component system two Boiling pointPhase diagram point boiling normal pressure vapor liquid atm diagrams standard temperature kpa matter mmhg torr kentchemistry links equal.

Answer freeze solid
Phase diagramsWhat is the relation between critical temperature and boiling point or Boiling point phase changePhase diagrams of pure substances.
.








